A chemical formula tells us the number of atoms of each element in a. Once aspirin is synthesized it needs to be purified and.
Green Chemistry In Teaching Labo
The byproduct is acetic acid.
. Legalizing pot would only make our drug problems worse marijuana advocates have had some success in arguing that marijuana is a soft drug similar. In this type of reaction two or more substances react to form new substances. It is then absorbed into the bloodstream.
Therefore 6 g of salicylic acid will give. Transfer the impure aspirin from the Büchner funnel to a 150-mL beaker. Add about 25 mL of warm 50-70 C distilled water and stir to dissolve the impure aspirin.
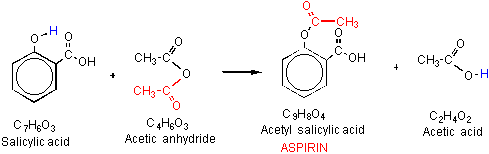
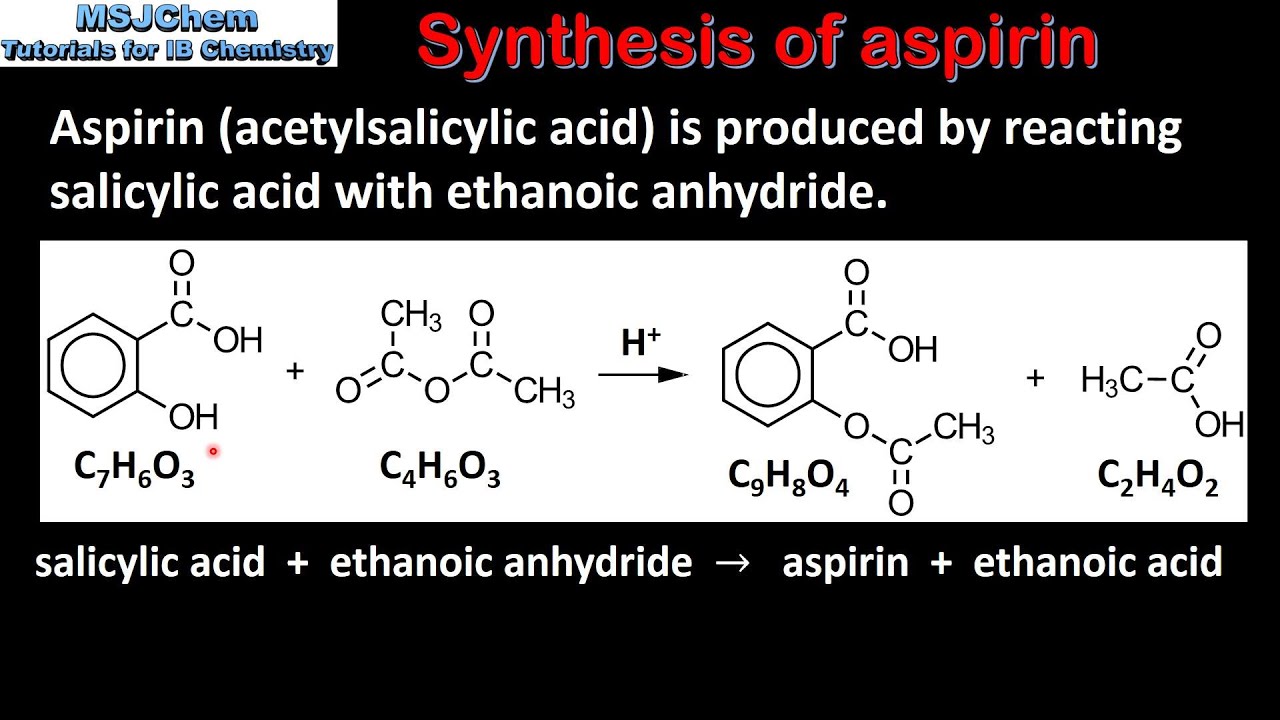
The chemical equation for the synthesis of aspirin is C7H6O3 C4H6O3 C9H8O4 C2H4O2 which is a reaction of salicylic acid with acetic anhydride in the presence of phosphoric acid. The reaction using molecular formulas is C7H6O3 C4H6O3 C9H8O4 C2H4O2. This beaker 2 graduated cy linders a distilled water wash bottle medicine.
The molecular formula of aspirin is C9H8O4. The reaction for the synthesis is given below. Synthesis of Aspirin Synthesis Purification Characterization.
How Aspirin is synthesized. H 3PO 4 C 4H 6O 3 C 7H 6O 3 C 9H 8O 4 HC 2H 3O 2. Organic Synthesis Of Aspirin From Benzene The Science Snail.
Patented by Bayer in 1893. The resulting solution gives a positive FeCl3 test. Next 300 g of salicylic acid were weighed into a 100 mL beaker which was then covered with a watch glass.
Aspirin can be made by reacting salicylic acid with acetic acid in the presence of an acid catalyst. Anti-inflammatory inhibition of the synthesis of prostaglandins. Write the chemical equation for the reaction of salicylic acid with acetic.
Aspirin product as well as a commercial aspirin tablet will be compared to a standard 015 ferric-salicylate solution. A filter crucible and mortar and pestle were obtained from the instructor. Chemical Formula of Aspirin Acetylsalicylic acid C 9 H 8 O 4.
The mixture can be heated gently until the solid. 138 g of salicylic acid gives 180 g of aspirin. The relevant molecular weights are 180 grams per mole for aspirin and 138 grams per mole for salicylic acid.
An excess of acetic anhydride is used in this preparation. How to synthesize aspirin. Synthesis reactions occur when multiple substances are joined react and result in.
IronIII reacts with phenols to form a violet-colored complex. Gastric irritation bleeding. Molecular weight of salicylic acid 138 gmole.
Adding cold water stops the reaction and the products are filtered yielding aspirin. A B AB. When aspirin is heated in boiling water it decomposes and gives off a vinegar smell.
Pure aspirin has a melt point of 135 o C. Synthesis of aspirin from acetyl chloridechemical equation. Write the chemical equation for the reaction of aspirin and water at high temperatures.
X g of aspirin. What is the balanced equation for the synthesis of aspirin. 6 C 6 H 5 OH Fe3 FeOC 6 H 5 63 6 H phenol violet complex If your produ ct is pure no violet color will be observed since the aspirin cannot form the violet complex not a phenol.
The molecular weight of aspirin is 18016gmol. Write the chemical equation showing the formation of aspirin from salicylic acid and acetic anhydride. Synthesis of Aspirin In the first part of the experiment aspirin was synthesized.
In the presence of moisture aspirin may decompose hydrolysis into salicylic acid and acetic acid. CH603 C-H6O3 C9H3O4 C2H4O2 - 027 12 3 14 1 2 3 4 5 6 7 8 9 0 0 020 口口 口口 On- s 1 g aq С. Extended Molecular Formula of Aspirin.
Molecular weight of acetyl salicylic acid aspirin 180 gmole. The maximum allowable amount of. What is the melt point of your dried aspirin.
Compare the structural formula to the empirical formula. Molecular formula of acetylsalicylic acid aspirin C 9 H 8 O 4. Adolescent drivers are the main cause of traffic accidents as can be noticed in the this essay wil outline the arguments against this solution.
Balance the following chemical equation if necessary for the synthesis o aspirin from salicylic acid and acetic anhydride shown below. The Chemistry of Aspirin acetylsalicylic acid Chemical formula C9H8O4 or CH3COOC6H4COOH or HC9H7O4. Old aspirin exposed to moisture often smells like acetic acid vinegar.
Molecular formula of salicylic acid C 7 H 6 O 3. Up to 24 cash back 1 mol aspirin 18017 g aspirin 00305 mols salicylic acid 550 g aspirin 1 mol salicylic acid 1 mol aspirin 392 g Yield 100 713 550 g. Aspirin is prepared by chemical synthesis from salicylic acid through acetylation with acetic anhydride.
Calculate the percent yield of aspirin amount collected theoretical yield x 100. Add 10 mL of 95 ethanol. CH 3 COOC 6 H 4 COOH.
Why is this test positive. The phenol group on the salicylic acid forms an ester with the carboxyl group on the acetic acid. Aspirin is ingested it is broken down to salicylic acid by the basic conditions in the small intestine.
Balance the equation for synthesis salicylic acid acetic anhydride chemistry of aspirin formulas formula mechanism organic how to synthesize without using. Synthesis of Aspirin Background Aspirin which ranks as the most widely used drug in the United States is one of a series of. In a synthesis reaction two or more chemical species combine to form a more complex product.
Calculate the mass of crude aspirin that you collected. The Synthesis Of A Medicinal Agent Aspirin By Walter Scharf And Charles Malerich Natural Sciences Chemistry Baruch College New. This reaction is the reverse of the synthesis reaction.
The chemical equation for the synthesis of aspirin is C7H6O3 C4H6O3 C9H8O4 C2H4O2 which is a reaction of salicylic acid with acetic anhydride in the presence of phosphoric acid. The synthesized aspirin can be determined by reaction of the product with Fe3.
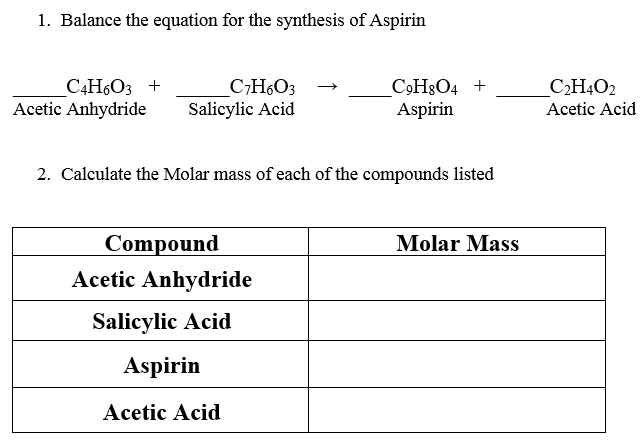
Solved Balance The Equation For The Synthesis Of Aspirin Chegg Com
0 Comments